Surfactants, Micelles and Asset Integrity
Surfactants
Surfactants are compounds that lower the interfacial tension between two liquids, between two phases, e.g. a solid and liquid, or two immiscible fluids; the word is a combination of the phrase surface-active agent. They are commonly used in oil & gas corrosion inhibitor formulations due to their ability to form film surfaces on the internal walls of production and transmission pipelines.
Quaternary amines, imidazolines, pyridiniums and phosphonate esters are commonly used in corrosion inhibition roles. When these components are present in sufficient concentrations, they can be detected as micelles by Anpera Technologies CoMic™ platform.
Surfactant Molecules
Corrosion Inhibitors
Surfactant in Brine
Micelles
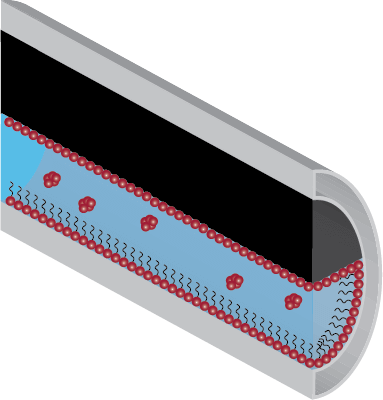
Micelles will form above a concentration known as the Critical Micelle Concentration (CMC) and at solution temperatures above the Krafft Point. Micelles are observed when all system demands have been met, or in other words, complete surface coverage has been attained.
Micelles & Optimal Dose
It is important to realise that commercial corrosion inhibitor products are very complex mixtures of chemical compounds. Thus, there is no such thing as “corrosion inhibitor residual” after the chemical is injected into a produced fluid system. The optimal concentration of corrosion inhibitor is ultimately an economic one, if it costs more to inject the inhibitor than the return in the extended life of the asset, it is not optimal
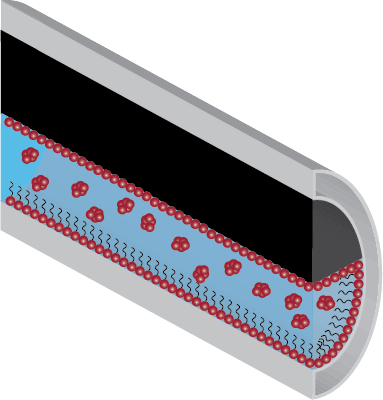
The presence of micelles originating from the dosing of corrosion inhibitor formulations signifies that all available surfaces within a system have been covered and that further addition may cause unforeseen issues such as separation problems and greater chemical costs.
At the inhibitor’s optimal concentration, the addition of more corrosion inhibitor will not significantly increase the inhibitor’s surface coverage of the steel further, since it will just go to form more - or larger - micelles.
CoMic™ has been tested in continuous flow loop tests and has shown a correlation between the minimal corrosion rate [LPR and weight-loss coupons] and formation of micelles in sample water. The simplicity of the test means that CoMic™ can be used to routinely assess the dosage of corrosion inhibitors and as a rapid assay on factors impacting chemical losses.
Optimally Dosed
Over Dosed
Optimally Dosed
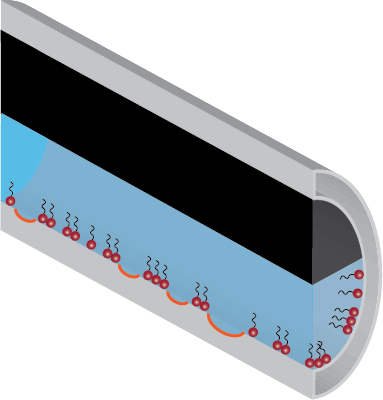
Micelle Index
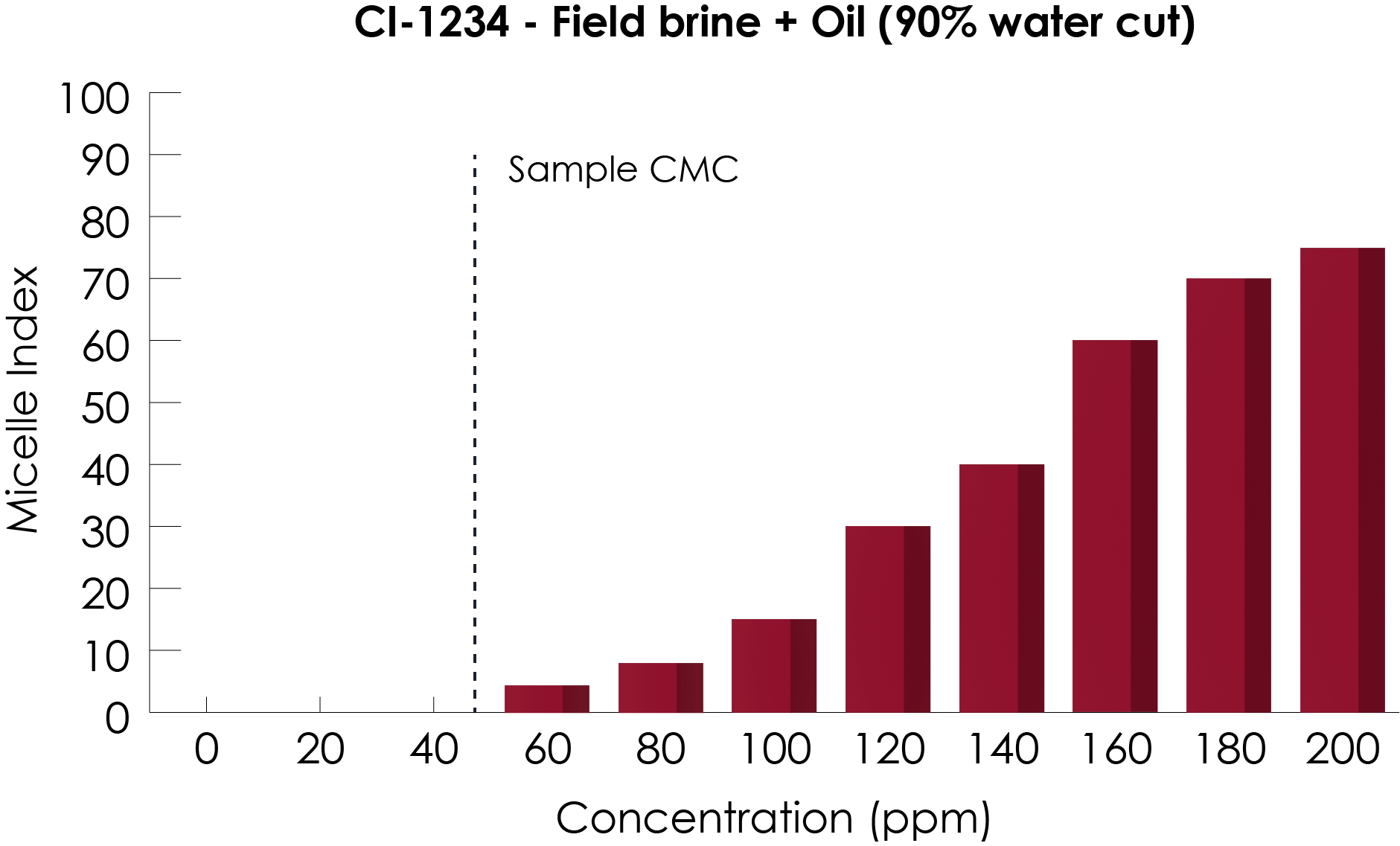
CoMic™ is a semi-quantitative assay; micelles are either present in a sample or not. When present, the amplitude of the CoMic™ signal allows an assessment of the general micelle population in a sample; this is reported as a dimensionless unit called the Micelle Index.
Samples in which no micelles are detected are assigned a value of zero, any samples in which micelles are detected will produce a micelle index value indicating the relative populations within the fluid. The upper limit of the scale, 100, is the maximum detectable result possible using CoMic™. Unlike amine residual tests, CoMic™ does not provide a quantified result in parts per million (ppm), as micelles are in a dynamic equilibrium with free monomer in solution.
In the example shown, a series of concentrations of corrosion inhibitor have been tested in a simulated field matrix. Results above zero show the presence of micelles in samples, with a clear trend in increasing population as sample concentration increases. The point at which samples transition from zero to a positive micelle index is known as the critical micelle concentration (CMC).
CoMic™ kits are available to calculate CMC in various matrices showing if a chemical is suitable for analysis in the field. On-Site test kits can then be used to confirm that micelles, and their relative populations, are present in production water.
Surfactant Availability
Surface area is not the only factor affecting injection rates of corrosion inhibitors. Conditions including partition and stability can greatly influence the availability of corrosion inhibitors within complex production and transport systems.
As systems age and conditions change dynamically, it is essential to monitor available chemical ensuring that system demands are met and that asset integrity is maintained. Changes to water cut, increased solids production or undetected deterioration to operational equipment can all impact the available chemical.
CoMic™ can rapidly detect micelle populations in production water, providing accurate and actionable data on-site.

